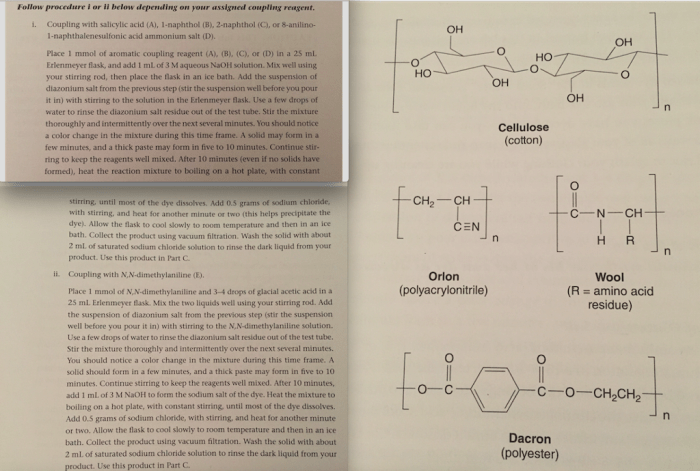
Calculate the amounts of salicylic acid 1-naphthol 2-naphthol – Calculating the amounts of salicylic acid, 1-naphthol, and 2-naphthol is a fundamental aspect of various scientific disciplines, ranging from pharmaceutical chemistry to environmental monitoring. These compounds possess unique properties and applications, and their precise quantification is essential for ensuring accuracy and reliability in research and industrial settings.
This article delves into the chemical structures, physical and chemical properties, and practical applications of salicylic acid, 1-naphthol, and 2-naphthol. It also provides a comprehensive comparison of these compounds, highlighting their similarities and differences.
Questions Often Asked: Calculate The Amounts Of Salicylic Acid 1-naphthol 2-naphthol
What are the key differences between salicylic acid, 1-naphthol, and 2-naphthol?
Salicylic acid is a monohydroxybenzoic acid, while 1-naphthol and 2-naphthol are monohydroxynaphthalenes. Salicylic acid has a carboxylic acid group, whereas 1-naphthol and 2-naphthol have hydroxyl groups.
What are the common applications of salicylic acid, 1-naphthol, and 2-naphthol?
Salicylic acid is used as an active ingredient in skincare products and medications for its anti-inflammatory and keratolytic properties. 1-naphthol is used as an intermediate in the production of dyes and pigments. 2-naphthol is used as a precursor for the synthesis of vitamin K and other pharmaceuticals.