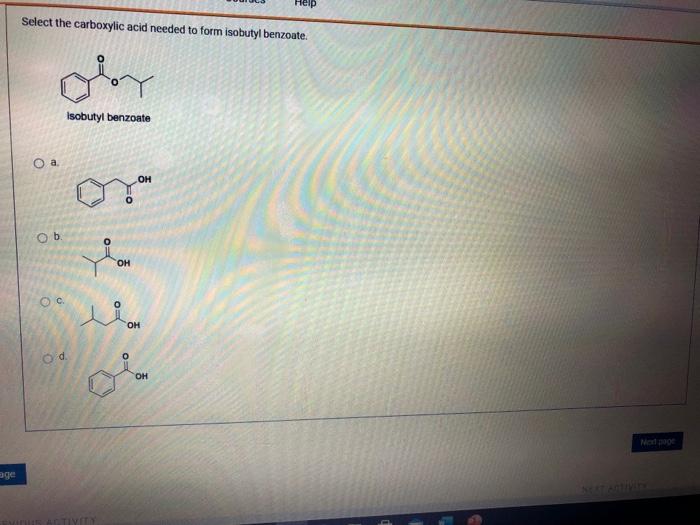
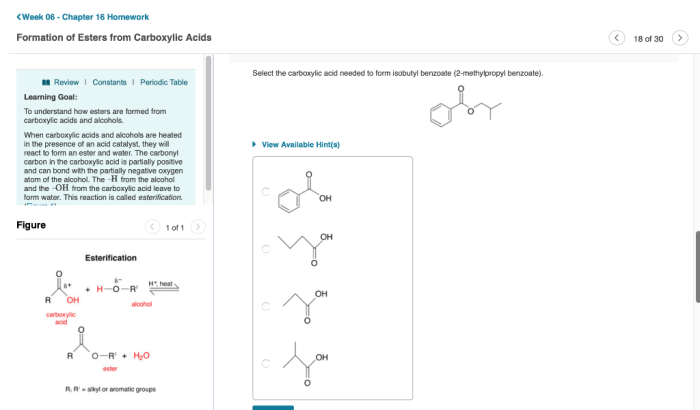
Select the carboxylic acid needed to form isobutyl benzoate – Selecting the appropriate carboxylic acid is a crucial step in the synthesis of isobutyl benzoate, as it directly impacts the yield, purity, and efficiency of the reaction. This guide provides a comprehensive overview of the factors to consider when choosing the carboxylic acid, explores the different synthesis methods, and discusses the reaction mechanism and optimization strategies.
By understanding the principles Artikeld in this guide, chemists can optimize the synthesis of isobutyl benzoate for various applications, ranging from fragrances and flavors to pharmaceutical intermediates.
Carboxylic Acid Selection for Isobutyl Benzoate Synthesis: Select The Carboxylic Acid Needed To Form Isobutyl Benzoate
The selection of the appropriate carboxylic acid is crucial for the successful synthesis of isobutyl benzoate. Several factors need to be considered, including the reactivity, availability, and cost of the acid.
Commonly used carboxylic acids for isobutyl benzoate synthesis include:
- Benzoic acid
- Acetic acid
- Propionic acid
- Butyric acid
- Isobutyric acid
The choice of carboxylic acid depends on the desired yield and purity of the final product. For example, benzoic acid is a good choice for high-yield reactions, while acetic acid is preferred for high-purity products.
Isobutyl Benzoate Synthesis Methods
Isobutyl benzoate can be synthesized using various methods, each with its advantages and disadvantages.
- Fischer esterification:This method involves reacting an alcohol with a carboxylic acid in the presence of an acid catalyst. It is a simple and cost-effective method, but it requires long reaction times and can produce side products.
- Swern oxidation:This method involves using dimethyl sulfoxide (DMSO) and oxalyl chloride to convert an alcohol to an ester. It is a versatile method that can be used with a wide range of alcohols, but it requires specialized reagents and can be hazardous.
- Mitsunobu reaction:This method involves reacting an alcohol with a carboxylic acid in the presence of triphenylphosphine and diethyl azodicarboxylate (DEAD). It is a mild and efficient method that can produce high yields of esters, but it requires expensive reagents.
The choice of synthesis method depends on the desired yield, purity, and cost requirements of the final product.
Reaction Mechanism and Optimization
The reaction mechanism for the formation of isobutyl benzoate involves a nucleophilic attack by the isobutanol oxygen on the carbonyl carbon of benzoic acid. This leads to the formation of a tetrahedral intermediate, which then collapses to form the ester bond.
The reaction rate and yield can be optimized by controlling the reaction temperature, catalyst concentration, and solvent choice. Higher temperatures and higher catalyst concentrations generally lead to faster reaction rates, but can also increase the formation of side products. The choice of solvent can also affect the reaction rate and yield, with polar solvents favoring the formation of the ester.
Isobutyl Benzoate Characterization and Applications
Isobutyl benzoate is a colorless liquid with a characteristic fruity odor. It is insoluble in water but soluble in organic solvents.
Isobutyl benzoate can be characterized using various analytical techniques, including gas chromatography-mass spectrometry (GC-MS), nuclear magnetic resonance (NMR) spectroscopy, and infrared (IR) spectroscopy.
Isobutyl benzoate has a wide range of applications, including:
- As a fragrance ingredient in perfumes and cosmetics
- As a flavoring agent in food and beverages
- As a solvent in the paint and coating industry
- As a plasticizer in the plastics industry
FAQs
What is the key factor to consider when selecting a carboxylic acid for isobutyl benzoate synthesis?
The key factor is the reactivity of the carboxylic acid, which is influenced by its structure and the presence of substituents.
What is the most efficient method for synthesizing isobutyl benzoate?
The most efficient method is the Fischer esterification, which involves heating the carboxylic acid with isobutyl alcohol in the presence of an acid catalyst.
How can the reaction yield of isobutyl benzoate be optimized?
The reaction yield can be optimized by using a stoichiometric excess of isobutyl alcohol, employing a suitable catalyst, and controlling the reaction temperature and time.