Delve into the captivating world of chemistry with the Basic Chemistry Chapter 2 Answer Key, your essential guide to mastering the fundamental concepts of this intriguing science. This comprehensive resource empowers you to unravel the mysteries of chemical equations, stoichiometry, and gas laws, unlocking a deeper understanding of the interactions that shape our world.
Prepare to embark on an enlightening journey as we explore the intricacies of limiting reactants, percent yield, solution chemistry, and acids and bases. Each topic is meticulously explained, providing you with a solid foundation to excel in your chemistry endeavors.
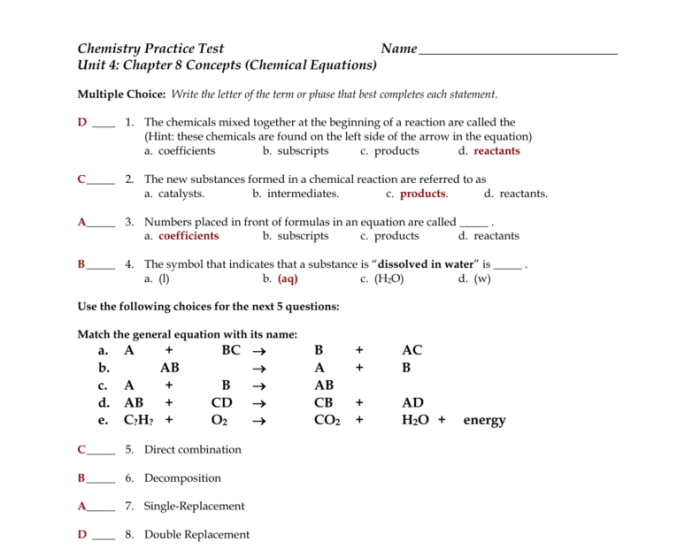
Chapter Overview
Chapter 2 of Basic Chemistry delves into the fundamental concepts of matter, its properties, and the changes it undergoes. It explores the composition and structure of atoms, the periodic table, and the behavior of elements in chemical reactions.
Key topics covered in this chapter include:
- The structure of atoms, including protons, neutrons, and electrons
- The arrangement of elements in the periodic table
- Chemical bonding and the formation of molecules
- Chemical reactions and the changes they produce
Chemical Equations

Chemical equations are symbolic representations of chemical reactions. They provide information about the reactants, products, and the stoichiometry of the reaction.
The reactants are the initial substances that undergo a chemical change, while the products are the final substances formed. Coefficients are used to balance the equation, ensuring that the number of atoms of each element is the same on both sides of the equation.
Balancing Chemical Equations
Balancing chemical equations is essential to ensure that the law of conservation of mass is upheld. Various methods can be employed to balance equations, including:
- Inspection method: By visually adjusting coefficients until the equation is balanced.
- Half-reaction method: Balancing redox reactions by separating the reaction into oxidation and reduction half-reactions.
- Matrix method: Using a system of linear equations to solve for the coefficients.
Stoichiometry
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It plays a crucial role in predicting the amounts of reactants and products involved in a given reaction and in determining the limiting reactant, which is the reactant that is completely consumed and limits the amount of product that can be formed.
Mass-Mass Calculations
In mass-mass calculations, we use the mole concept to convert between the mass of a reactant or product and the number of moles. The molar mass of a substance is its mass per mole, and it allows us to determine the number of moles of a substance present in a given mass.
For example, if we have 10.0 g of sodium chloride (NaCl), we can convert this to moles using its molar mass (58.44 g/mol):
“`
0 g NaCl × (1 mol NaCl / 58.44 g NaCl) = 0.171 mol NaCl
“`
Mole-Mole Calculations
Mole-mole calculations involve converting between the number of moles of reactants and products using the stoichiometry of the balanced chemical equation. The coefficients in the balanced equation represent the mole ratio between the reactants and products.
For example, consider the following reaction:
“`
H2+ O 2→ 2 H 2O
“`
This equation tells us that 2 moles of hydrogen gas (H 2) react with 1 mole of oxygen gas (O 2) to produce 2 moles of water (H 2O). Therefore, if we have 0.5 mol of H 2, we can calculate the number of moles of O 2required using the mole ratio:
“`
5 mol H2× (1 mol O 2/ 2 mol H 2) = 0.25 mol O 2
“`
Volume-Volume Calculations
Volume-volume calculations involve converting between the volumes of gases involved in a reaction using the ideal gas law. The ideal gas law states that the volume of a gas is directly proportional to the number of moles of gas and inversely proportional to the pressure and temperature.
For example, if we have 1.0 L of hydrogen gas (H 2) at standard temperature and pressure (STP), we can convert this to moles using the ideal gas law:
“`PV = nRT
0 L × 1 atm = n × 0.0821 L·atm/(mol·K) × 273 K
n = 0.040 mol H 2“`
Limiting Reactants: Basic Chemistry Chapter 2 Answer Key
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, thus limiting the amount of product that can be formed. The other reactants are present in excess and do not limit the reaction.
To determine the limiting reactant, we compare the moles of each reactant to the stoichiometric ratio in the balanced chemical equation. The reactant with the smallest mole ratio to its stoichiometric coefficient is the limiting reactant.
Example: Limiting Reactant Calculation
Consider the reaction between hydrogen (H 2) and oxygen (O 2) to form water (H 2O):
2H 2+ O 2→ 2H 2O
If we have 2 moles of H 2and 1 mole of O 2, we can calculate the mole ratios:
H 2: 2 moles / 2 = 1 O 2: 1 mole / 1 = 1
Since the mole ratio of O 2is smaller than that of H 2, O 2is the limiting reactant.
Applications of Limiting Reactant Calculations
- Predicting the maximum amount of product that can be formed in a reaction.
- Determining the efficiency of a chemical process.
- Designing experiments to optimize product yield.
Percent Yield
Percent yield is a measure of the efficiency of a chemical reaction. It is defined as the ratio of the actual yield of a product to the theoretical yield, multiplied by 100%. The theoretical yield is the maximum amount of product that can be obtained from a given amount of reactants, based on the stoichiometry of the reaction.
Percent yield can be affected by a number of factors, including the purity of the reactants, the reaction conditions, and the presence of side reactions. To improve percent yield, it is important to use pure reactants, optimize the reaction conditions, and minimize side reactions.
Factors Affecting Percent Yield
- Purity of reactants: Impurities in the reactants can react with each other or with the desired product, reducing the yield of the desired product.
- Reaction conditions: The temperature, pressure, and solvent used in a reaction can affect the rate of the reaction and the yield of the product.
- Side reactions: Side reactions are unwanted reactions that can occur in addition to the desired reaction. Side reactions can consume reactants and reduce the yield of the desired product.
Improving Percent Yield
- Use pure reactants: The use of pure reactants will help to minimize the occurrence of side reactions and improve the yield of the desired product.
- Optimize reaction conditions: The reaction conditions can be optimized to increase the rate of the desired reaction and minimize the occurrence of side reactions.
- Minimize side reactions: Side reactions can be minimized by using selective catalysts, adding inhibitors, or changing the reaction conditions.
Gas Laws

Gas laws describe the behavior of gases under various conditions. These laws provide a quantitative understanding of the relationship between pressure, volume, temperature, and the number of moles of a gas.
Boyle’s Law
Boyle’s law states that the pressure of a gas is inversely proportional to its volume at constant temperature. Mathematically, it can be expressed as P₁V₁ = P₂V₂. This law implies that as the volume of a gas increases, its pressure decreases, and vice versa.
Charles’s Law
Charles’s law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure. Mathematically, it can be expressed as V₁/T₁ = V₂/T₂. This law implies that as the temperature of a gas increases, its volume also increases, and vice versa.
Ideal Gas Law
The ideal gas law combines Boyle’s law and Charles’s law and introduces the concept of the number of moles of a gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant (0.0821 L⋅atm/(mol⋅K)), and T is temperature in Kelvin.
Gas Law Calculations
Gas laws can be used to solve various problems involving pressure, volume, temperature, and number of moles. For example:
- To calculate the pressure of a gas if its volume is increased:
- To calculate the volume of a gas if its temperature is decreased:
- To calculate the number of moles of a gas if its pressure and volume are known:
P₁V₁ = P₂V₂If V₁ = 1 L, P₁ = 1 atm, and V₂ = 2 L, then P₂ = 0.5 atm.
V₁/T₁ = V₂/T₂If V₁ = 1 L, T₁ = 300 K, and T₂ = 200 K, then V₂ = 0.67 L.
PV = nRTIf P = 1 atm, V = 1 L, and T = 298 K, then n = 0.04 mol.
Solution Chemistry

Solution chemistry involves the study of homogeneous mixtures called solutions, which consist of a solute (the substance dissolved) and a solvent (the dissolving medium). Solutions are classified into various types based on their physical and chemical properties, such as aqueous solutions, non-aqueous solutions, electrolytes, and non-electrolytes.
Solution Concentration, Basic chemistry chapter 2 answer key
The concentration of a solution is a measure of the amount of solute present in a given amount of solvent or solution. Common concentration units include molarity (M), molality (m), and mass percentage (%).
- Molarity (M): Molarity expresses the number of moles of solute per liter of solution.
- Molality (m): Molality represents the number of moles of solute per kilogram of solvent.
- Mass Percentage (%): Mass percentage indicates the mass of solute present in 100 grams of solution.
These concentration units are used to quantify the composition of solutions and to perform calculations involving solution stoichiometry.
Acids and Bases

Acids and bases are fundamental concepts in chemistry that describe substances with distinct properties and reactivity. Understanding their behavior and interactions is crucial in various chemical processes and applications.
The Arrhenius theory defines acids as substances that, when dissolved in water, release hydrogen ions (H+) and bases as substances that release hydroxide ions (OH-). The Brønsted-Lowry theory expands on this definition, describing acids as proton (H+) donors and bases as proton acceptors.
Properties of Acids and Bases
- Acids are typically sour, corrosive, and can react with metals to produce hydrogen gas.
- Bases are bitter, slippery, and can neutralize acids, forming salts.
Acid-Base Reactions
Acids and bases react with each other in a neutralization reaction, forming a salt and water.
Acid + Base → Salt + Water
These reactions are important in many chemical processes, such as the production of fertilizers and the regulation of pH in biological systems.
Applications of Acids and Bases
- Acids are used in the production of fertilizers, dyes, and batteries.
- Bases are used in the production of soaps, detergents, and paper.
- Acid-base reactions are essential for maintaining the pH balance in biological systems, such as the human body.
FAQs
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the conservation of mass during a chemical reaction.
How do I determine the limiting reactant in a reaction?
To identify the limiting reactant, compare the mole ratio of each reactant to its stoichiometric coefficient. The reactant with the smallest mole ratio is the limiting reactant, as it will be completely consumed, limiting the amount of product formed.
What factors can affect the percent yield of a reaction?
The percent yield can be influenced by factors such as side reactions, incomplete reactions, and loss of product during purification.